The Theory of pH Measurement
Lý thuyết về nguyên lý đo PH
pH is a measure of the acidity or alkalinity of a water solution. The acidity or alkalinity of a water solution is determined by the relative number of hydrogen ions (H+) or hydroxyl ions (OH-) present. Acidic solutions have a higher relative number of hydrogen ions, while alkaline (also called basic) solutions have a higher relative number of hydroxyl ions. Acids are substances which either dissociate (split apart) to release hydrogen ions or react with water to form hydrogen ions. Basesare substances that dissociate to release hydroxyl ions or react with water to form hydroxyl ions.


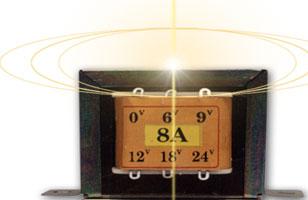 Tính toán quấn máy biến áp 1 pha tần số 50Hz
Tính toán quấn máy biến áp 1 pha tần số 50Hz 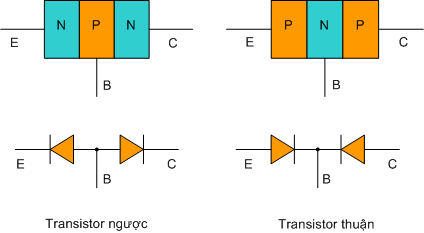 Cấu tạo, nguyên tắc hoạt động của Transitor
Cấu tạo, nguyên tắc hoạt động của Transitor  Nguyên lý và sử dụng nguồn xung hay bộ biến đổi nguồn DC-DC
Nguyên lý và sử dụng nguồn xung hay bộ biến đổi nguồn DC-DC  Làm LED trái tim với 8501
Làm LED trái tim với 8501  Ký hiệu, Hình dạng, kiểm tra, Xác định chân Transitor
Ký hiệu, Hình dạng, kiểm tra, Xác định chân Transitor 

